
Chemists back in the 18th century stumbled onto pyrogallol while tinkering with tannic acids. Scheele managed to isolate gallic acid, and a little later, pyrogallol turned up on the scene as a white crystalline powder. The earliest uses revolved around hair dyeing, photography, and colorimetric oxygen detection—long before anyone could rattle off chemical structures from memory. Those early chemists noticed that the substance quickly darkened on exposure to air, and that feature carved out a crucial role in analytical chemistry, especially before electronic oxygen sensors. For a while, pyrogallol held court in the darkrooms of the world’s photographers, especially for developing silver-based films, until digital cameras swept the process aside.
Pyrogallol shows up as a white or slightly gray crystalline solid with an ability to turn brown in air, as it grabs hold of oxygen. Chemists know it better as 1,2,3-trihydroxybenzene, and it’s typically found in technical grade or purer forms up to 99.5%. Its solubility in water and alcohol helps for easy application in the lab, but its reactive nature keeps people cautious. People still use it in specialty dye production and for some kinds of organic synthesis, but you won’t find it on every shelf outside research, niche manufacturing, or specialist chemical suppliers due to safety protocols and limited consumer demand.
Pyrogallol comes with a melting point near 132°C, which keeps it stable at room temperature. This powder is distinctly soluble in water, ethanol, and ether, which makes cleanup and application manageable, though it reacts fast with oxidizers. The benzene ring with three hydroxyl groups gives the compound its chemical punch, which means it easily loses hydrogen to oxidizing agents, shifting color fast as it oxidizes to purpurogallin—a reaction that scientists have tracked for decades. The pH in aqueous solutions leans toward acidity, and exposure to open air pushes it to degrade, so storing it away from light and moisture preserves its integrity for reliable results.
Manufacturers label pyrogallol by its purity grade, batch number, and standard hazard warnings in accordance with global regulations. The standard UN number 2811 flags toxic solid, organic, n.o.s., which demands locked storage and handling protocols. Labels usually specify handling conditions and include safety pictograms for acute toxicity, environmental hazard, and the need for gloves, goggles, and fume hoods. Technical datasheets break down assay percentage, impurities, water content, and recommended shelf life, which helps laboratories maintain traceability and compliance with both local and international chemical standards.
Traditional preparation starts from gallic acid, itself obtained from natural plant materials such as oak galls or tara pods. Heating gallic acid in the absence of air triggers decarboxylation, dropping carbon dioxide and leaving behind pyrogallol. The equipment covers strong glassware or steel, heating blocks, and closed reaction setups to avoid oxygen exposure. After the reaction finishes, cooling, recrystallization, and purification cycles follow. Chemical suppliers who scale up for industry use continuous reactors and larger batch processes, always dictating closed systems for worker safety and product purity.
The three hydroxyl groups on the benzene core open a world of transformation possibilities. Pyrogallol undergoes oxidative coupling, producing pigments or analytical dyes under mild conditions. Treated with strong bases or oxidizers, it gives rise to purpurogallin and other colored derivatives, which turn up in pigment or chemical indicator work. Reduction, acetylation, and etherification of the hydroxyls provide building blocks for organic synthesis. Analytical chemists still use its reactivity with oxygen for precise oxygen measurement, especially in glovebox and laboratory gas purity applications. The versatility in modifying aromatic hydroxyls sets it apart, making it a useful intermediate for pharmaceutical and dye manufacturing.
Beyond the International Union of Pure and Applied Chemistry name “1,2,3-trihydroxybenzene,” most chemists refer to it as pyrogallol. Other names floating around the literature include pyrogallic acid, benzene-1,2,3-triol, 1,2,3-benzenetriol, and C.I. 76560. Chemical suppliers and specialty distributors keep all these tags in their catalogs to help professionals cross-reference and avoid confusion. Deliberate naming supports hazard tracking, safety data accuracy, and international supply chain workflows, which depend on tight chemical nomenclature.
Anyone working with pyrogallol faces skin, eye, and respiratory hazards if exposed, so closed systems, gloves, protective eyewear, and respirators form the first line of defense. The powder and vapors irritate tissue and pose poisoning risks if ingested, inhaled, or absorbed through skin, and spills require containment and chemical neutralization agents on standby. Emergency wash stations, fume hoods, and strict labeling cut down dangerous exposure. Modern facilities that use pyrogallol for process chemistry or oxygen measurement maintain clear operating procedures, inspections, and medical monitoring for chronic exposure—even at low levels. The European Chemicals Agency (ECHA) and United States Occupational Safety and Health Administration (OSHA) both demand special attention to disposal, calling for incineration by licensed hazardous waste processors, with no exceptions for drain or landfill disposal.
Industry and laboratory use dominate pyrogallol’s footprint. Chemical manufacturers blend it into hair dyes, inks, and colorants when the deep brown or black shades are needed, though modern safety standards have restricted personal care applications. Analytical chemists still measure dissolved oxygen by timing how quickly pyrogallol darkens and quantifying the oxygen uptake, a method that works well in gloveboxes or labs lacking digital O2 sensors. Histologists and specialty pigment makers value its oxidation products in staining protocols that highlight cell walls, nuclei, and connective tissue with vivid contrast. In metallurgy or environmental analysis, the chemical helps pinpoint oxygen or certain reducing agents, which keeps legacy methods alive in some regulatory and academic labs.
Recent research efforts focus on green synthesis, aiming to cut solvent waste and hazards during pyrogallol production. Enzyme-catalyzed routes and sustainable plant sources are under investigation, as the drive for environmentally conscious processes rises across the chemical industry. In organic electronics, pyrogallol derivatives show promise as building blocks for new conductive polymers, explored for use in displays or flexible circuitry. Cross-disciplinary teams play with its antioxidant properties, testing pharmaceutical leads for potential inflammation or oxidative stress treatments. Teams working in cancer biology dig through its pro- and antioxidant dual activity, eyeing lead compounds that moderate cell cycling and apoptosis. As widespread chemical screening platforms reach new scales, expect to see more modifications and applications show up both in patents and the peer-reviewed literature.
Toxicologists cite pyrogallol as acutely toxic at high exposures, both for humans and aquatic life. The material triggers methemoglobinemia, oxidative stress, and cell lysis, especially after ingestion or unprotected handling—underscored by case reports dating back to the early 20th century. Chronic exposure may affect blood chemistry and organ function, which pushes for additional protections beyond what’s listed on basic Safety Data Sheets. Its high reactivity with oxygen sometimes gets leveraged in topical antiseptic formulations, but only at low, well-studied concentrations. The chemical’s acute and chronic toxicity keeps regulatory agencies on alert for any uptick in production or accidental release, and new studies often explore mechanisms of DNA and protein alteration in hopes of establishing safer exposure thresholds.
Despite the health and environmental warnings, pyrogallol continues to grab interest in research and specialized manufacturing. Regulatory reforms shaping chemical supply and disposal push innovation toward less hazardous analogs, but the unique reactivity and dyeing ability keep it involved in niche sectors. Biomedicine and organic electronics labs look to the compound for new material leads, especially those that balance redox activity with safety—though tough safety and labeling rules shape every step from synthesis to disposal. With artificial intelligence and automated chemical discovery on the rise, expect more nuanced, targeted research into pyrogallol derivatives for antioxidant therapies, conductive polymers, and low-impact dyes. Existing obstacles around toxicology and environmental buildup drive steady exploration and improvement, balancing the benefits of powerful chemistry against the need for safety and sustainability.
Pyrogallol has been around for ages. In the lab, brown bottles of it sit on shelves along with other old standbys, and it never makes the headlines. Still, this white powder remains useful. Its real value shows up in the way it eats up oxygen. Chemists and students learn early that if you need something to mop up oxygen from the air, you reach for pyrogallol. Pour some into a beaker, add a little alkaline solution, and you watch it go brown as it does its job. That skill matters in industries and labs that want an easy way to measure or remove oxygen from air or solutions.
At university, I remember loading up simple glass tubes with pyrogallol to test for trace oxygen. Plant scientists use this trick all the time. They want to know how much oxygen leaves make after photosynthesis or how much a soil sample breathes out. The color change is a clear sign of how much gas you have. Titling this a “sophisticated” technique would be a stretch. It stays popular because it comes cheap and most labs trust what they see. In this age of digital sensors, pyrogallol offers an approachable, low-tech backup — and sometimes, results hold just as much weight.
Those who still shoot with film cameras know the story. In the early days of photography, developers often relied on pyrogallol. It helped bring out images with crisp lines and good contrast. These days, few shops still stock black-and-white film, and even fewer process it with old-fashioned chemicals, but pyrogallol’s fingerprint lingers in the darkroom for special techniques. Enthusiasts claim it creates a unique tonal range, so rare prints sometimes fetch high prices among collectors.
Doctors once used pyrogallol to treat certain skin conditions like psoriasis. That fell out of favor after safer remedies arrived. Still, research labs find the compound handy for making dyes and stains that help show cellular structures under a microscope. While other chemicals have taken center stage, pyrogallol remains a solid choice during early research phases or for those on tight budgets.
Every time I’ve handled pyrogallol, the label shows a little skull — and that’s a real sign to keep it away from food, skin, and lungs. It stains, irritates, and if swallowed, it can be deadly. Factory workers in chemical plants used to handle loads of the stuff, and stories of exposure floated around. Working safely means protective gloves, goggles, and good ventilation. Waste disposal is another challenge. Poured down the drain, it poses risks to water supplies. Industry groups recommend collecting and neutralizing old solutions before safe disposal, and stricter rules now guide big users.
Safer, smarter ways to remove and measure oxygen keep popping up. Portable meters and new non-toxic chemicals promise less hassle for those with money to spend. Yet, pyrogallol sticks around, often because it’s simple and costs less. The legacy of this old compound reminds us that proven tools don’t disappear overnight, especially in places where budgets run tight or equipment breaks down. Keeping safety in mind, its story continues, quietly helping scientists and tinkerers alike do more with less.
Pyrogallol’s not a household item, but in research labs and some industries, people rely on it for things like photography, hair dye, and as a reagent in chemical analysis. Its roles range from scavenging oxygen in chemical reactions to acting as an antioxidant in specialized applications. Handling pyrogallol doesn’t just mean following routine lab rules. It demands focused attention because this material can cause serious health issues if not managed carefully.
Pyrogallol can enter the body through skin contact, inhalation, or accidental ingestion. Its dust or solutions might burn skin, damage eyes, and, when inhaled, irritate respiratory passages. Even short-term exposure, from an accidental splash or a waft of dust, can bring on headaches, dizziness, and even nausea. Long-term or repeated exposure won’t just leave a rash; people have developed dermatitis and even more severe conditions over time.
I remember working with phenols of a similar type during a university project. I had gloves, but a small tear went unnoticed, and a tiny spot left my fingers red for days. It’s surprising how little contact can have big effects. That lesson stuck with me and shaped a “glove up and slow down” rule any time I work with reactive chemicals like pyrogallol.
Ventilation tops the list. If a lab doesn’t have a working fume hood, pyrogallol shouldn’t touch a bench. Sufficient airflow and local exhaust draw away vapors and fine dust. Air quality matters not just for comfort but because controlling dust and vapor reduces accidental exposure. Respiratory protection, like a fitted N95 mask, might come into play for weighing or transferring the powder.
Choosing the right gloves means skipping thin latex and turning to nitrile, which handles phenolic chemicals with far less risk of tearing or breakthrough. Goggles shield against stray splashes. Closed-toe shoes and lab coats become an easy habit—no one wakes up planning to spill, but even the most experienced chemist drops a beaker sometimes.
Spills are never entirely avoidable, but handling them quickly and confidently cuts down on danger. Spill kits for chemicals need to be close by, well-stocked, and checked often. If pyrogallol lands on a surface, scooping it up with disposable towels and cleaning with copious water clears the area. Absorbents and neutralizers tailored for organic compounds make cleanup more effective.
Disposing of waste deserves extra attention. Pyrogallol can’t be dumped down a drain. Special waste containers, separate from regular trash, eliminate cross-contamination. Facilities with hazardous waste pickup become your best friend in keeping labs and surroundings safe.
Even with strict habits, emergencies can happen. Fast access to eye wash stations, showers, and trained colleagues saves critical minutes in a crisis. Safety data sheets need to be easy to find, not buried under other paperwork. Regular training—walking through what to do in a spill or exposure—prepares everyone, not just the person directly working with pyrogallol.
Building a safety culture does more than following rules. Labs with open communication, regular checks on protective gear, and a focus on good habits stay safer. Investing in quality gear, never skimping on training, and support from supervisors set the scene for responsible chemical handling. My experience, shared with dozens of colleagues, shows that taking these steps seriously keeps accidents minor, not life-changing.
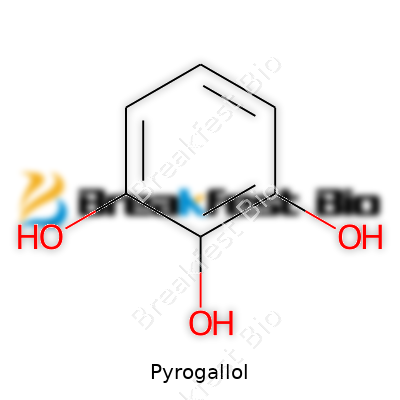
Pyrogallol often grabs attention in labs and photography darkrooms, but its impact stretches far beyond. The chemical formula stands as C6H6O3, meaning one molecule carries six carbon atoms, six hydrogen atoms, and three oxygen atoms. Knowing this formula already tells a story about the arrangement and potential reactivity of the molecule. Chemists see it as a trihydroxybenzene, where three hydroxyl groups cling to a benzene ring at positions 1, 2, and 3. This setup shapes both physical behavior and how scientists use it.
In textbooks, the structure of pyrogallol appears as a classic hexagonal ring, like a honeycomb representing benzene. Three —OH groups branch off at carbons next to each other, lining up at the 1,2,3 spots. It’s not just an aesthetic feature. This close arrangement gives the molecule its intense reactivity and high love for hydrogen bonding. Picture it forming instant chemistry with water and other polar solvents. I remember the first time I saw it under the microscope in an organic lab; crystals dissolved so readily when dropped in water it felt like magic, but it was just the science of those –OH groups at work.
Pyrogallol turns heads for more than its basic chemistry. In the 1800s, darkroom workers mixed it with silver nitrate to develop photographs. That’s because those three hydroxyl groups speed up the reduction of silver ions. Even now, labs use it as a benchmark to measure oxygen thanks to its rapid reaction with O2. The resulting color change signals oxygen’s presence, which helps in both academic and industrial setups. Some medical tests also use this unique reacting power.
Though its benefits are clear, there’s a flip side. Pyrogallol’s aggressive chemical traits mean skin contact or inhalation calls for caution. Reports show it can cause skin blotches, headaches, or worse if handled carelessly. Its tendency to auto-oxidize creates potential hazards, especially if stored in open air or near heat. Good lab habits always pay off: gloves, goggles, and capped bottles keep both students and professionals safe. From my own experience, an old professor would hammer home the value of safety with every live demonstration, showing what a carelessly handled bottle could do.
Questions about safe handling and environmental risk always raise controversy. Reports from OSHA and the National Institute for Occupational Safety and Health have pointed out that regulations must be clear. Using proper ventilation, secure labeling, and closed systems protects workers. For waste, strong oxidizers or incineration break it down, but only in factories with the right filters and environmental standards. With its role as both a valuable reagent and a potential hazard, thoughtful usage becomes non-negotiable.
Future chemistry will likely keep refining pyrogallol’s production and uses. Researchers already study ways to substitute less-hazardous compounds in some lab routines. Still, pyrogallol sticks around because its structure gives unmatched reactivity, and for certain niche uses, no alternative quite measures up. Understanding it—down to the atomic level—lets us use it wisely, safely, and for positive progress in science and industry.
Pyrogallol has a reputation for being sensitive to both air and light. This chemical, used in fields from photography to medicine, reacts fast when it touches oxygen. If you’ve ever opened a bottle and found the contents turning brown or even black, that’s not just disappointing—it’s risky for any process counting on its purity. Even casual handling, like leaving a cap a bit loose, shortens its shelf life.
Poor storage doesn’t just wreck the chemical. In my own university lab days, I saw some rookie mistakes cost us a whole order within weeks. Besides the waste, there’s safety on the line. Pyrogallol, as it breaks down, can give off irritating vapors and sometimes generates enough pressure to pop a poorly designed container. There are reports of serious messes from ignoring basic storage protocols: chemical spills, ruined experiments, sometimes even skin or eye irritation.
Glass works best for storage. It doesn’t react—unlike certain plastics or metals. A brown glass bottle blocks out most light, cutting down on those photo-chemical reactions that turn pyrogallol into sludge. Keep that bottle tightly capped. I’ve seen more than one brand-new shipment go bad just because the seal failed.
For temperature, shoot for cool but not freezing. Ideally, stick with a dry, climate-controlled cupboard. Humidity speeds up degradation, and high temperatures can kickstart chemical reactions that turn pyrogallol to useless gunk. Try to store it away from heat sources, sunlight, or any area where temperatures swing.
Don’t store it near acids, bases, or oxidizers. In larger setups, a separate storage cabinet lined with spill trays is smart policy. No one wants to clean up a sticky, toxic puddle in a tight chemical storage room. Routine checks help: look for color shifts, clumps, or moisture inside the bottle. Once the powder yellows or browns, toss it. No process should gamble on compromised product, especially in sensitive applications like hair dye or lab work.
Gloves and eye protection are basic protocol. Storage areas get labeled clearly—no confusion, no accidents. In workplaces with strict safety standards, chemical inventory logs track who takes what, and returns get inspected. This sounds strict, but after seeing a shelf collapse from an unmarked, corroded bottle, you won’t want to cut corners.
Switching to smaller bottles reduces spoilage. Buying in bulk saves money but breaks down fast once opened. Spreading storage across several sealed containers means mistakes cost less. In industrial setups, desiccant packs inside secondary containers help control moisture—even in humid climates.
Some labs use nitrogen or another inert gas to fill the headspace in storage bottles. This prevents oxygen from ruining the chemical before it gets used up. Such practices work especially well for researchers who go through a lot of stock.
Smart storage controls—regular training, careful labeling, airtight containers, and ongoing vigilance—make pyrogallol less of a liability and more of a useful tool for the job at hand.
Pyrogallol isn’t a household name, yet it turns up in places that matter—labs, older photographic processes, hair dye manufacturing, even some types of medical testing. This compound, also known as 1,2,3-trihydroxybenzene, has a reputation for punching above its weight class in both chemistry and toxicity. Anyone who has handled it in university labs will recall the warnings posted above the scale and the conversations about proper ventilation. Pyrogallol stains fingers brown and demands respect, but the real hazard runs deeper than discoloration.
Skin contact with pyrogallol can cause irritation, but the story doesn’t stop at redness or rashes. Inhalation and ingestion present bigger dangers. A study published in the journal Occupational Medicine describes cases where exposure led to deep tissue burns and systemic poisoning. Workers who handled pyrogallol without proper gloves and masks complained of headaches and felt nauseous. The compound affects blood by oxidizing hemoglobin, causing symptoms like dizziness and shortness of breath. Long-term, chronic exposure might increase the risk for more serious conditions, including methemoglobinemia, which undermines oxygen transport in the body. Researchers at the European Chemicals Agency classify pyrogallol as a hazardous substance based on these effects.
Pyrogallol's environmental footprint raises red flags as well. The chemical leeches quickly into soil and water sources. Fish and aquatic life show signs of toxicity when exposed, with laboratory data pointing to reduced growth and neurological impairment even at lower concentrations. The U.S. Environmental Protection Agency’s Ecotox database lists pyrogallol as highly hazardous to aquatic organisms. Rapid breakdown in soil doesn’t offer complete peace of mind. Byproducts of pyrogallol breakdown—including phenols—carry their own set of risks, adding layers of complexity to local water management.
Old college habits still stick, and pyrogallol taught me that personal protective equipment matters. I remember splashes on a benchtop prompting a scramble for neutralizers and fume hoods. Failing to take these measures often led to immediate nose and throat irritation for anyone in the room. In workplaces dealing with chemicals, practice demands airtight procedures—closed systems, exhaust fans, spill kits, and rigorous training for everyone involved. Industry reports back this experience: improper disposal of pyrogallol and similar compounds leads to long-term soil and groundwater contamination. A handful of industrial spills, documented in environmental review articles, show how cleanup carries a hefty price tag for both the company and surrounding residents.
Switching to safer alternatives makes sense where possible. Some photographic processes have already ditched pyrogallol in favor of less aggressive developing agents. For places where its use can’t be avoided, strict air monitoring and regular health screenings help keep workers safe. Treatment plants using advanced oxidation can break down leftover chemicals before discharge, reducing the risk posed to streams and lakes. Community transparency, frequent inspections, and tougher waste regulations all help prevent avoidable harm. In any setting—whether academic, industrial, or commercial—the lesson sticks: chemicals like pyrogallol earn their hazardous label, so handling them with care protects both the people around and the world outside.
| Names | |
| Preferred IUPAC name | benzene-1,2,3-triol |
| Other names |
1,2,3-Trihydroxybenzene
Pyrogallic acid Benzene-1,2,3-triol |
| Pronunciation | /paɪˈrɒɡəˌlɒl/ |
| Identifiers | |
| CAS Number | 87-66-1 |
| Beilstein Reference | 1368602 |
| ChEBI | CHEBI:16044 |
| ChEMBL | CHEMBL1406 |
| ChemSpider | 1046 |
| DrugBank | DB03793 |
| ECHA InfoCard | 100.004.454 |
| EC Number | 205-749-9 |
| Gmelin Reference | 52927 |
| KEGG | C01432 |
| MeSH | D011693 |
| PubChem CID | 1060 |
| RTECS number | UZ7560000 |
| UNII | 3Z5A34W8Y9 |
| UN number | UN1500 |
| Properties | |
| Chemical formula | C6H6O3 |
| Molar mass | 126.11 g/mol |
| Appearance | White crystalline solid |
| Odor | odorless |
| Density | 1.46 g/cm³ |
| Solubility in water | very soluble |
| log P | 0.76 |
| Vapor pressure | 0.00016 mmHg (25°C) |
| Acidity (pKa) | 9.05 |
| Basicity (pKb) | 4.1 |
| Magnetic susceptibility (χ) | χ = -62.0·10⁻⁶ cm³/mol |
| Refractive index (nD) | 1.460 |
| Viscosity | 1.792 cP (at 25 °C) |
| Dipole moment | 1.694 D |
| Thermochemistry | |
| Std molar entropy (S⦵298) | 151.0 J·mol⁻¹·K⁻¹ |
| Std enthalpy of formation (ΔfH⦵298) | -452.9 kJ/mol |
| Std enthalpy of combustion (ΔcH⦵298) | -1338 kJ mol⁻¹ |
| Pharmacology | |
| ATC code | D11AX12 |
| Hazards | |
| Main hazards | Toxic if swallowed, in contact with skin or if inhaled; causes severe skin burns and eye damage; may cause respiratory irritation. |
| GHS labelling | GHS02, GHS06, GHS08 |
| Pictograms | GHS06,GHS09 |
| Signal word | Danger |
| Hazard statements | H301, H311, H331, H373, H400 |
| Precautionary statements | P210, P280, P302+P352, P305+P351+P338, P310, P322, P362+P364, P370+P378, P403+P233, P501 |
| NFPA 704 (fire diamond) | 3-2-2 |
| Flash point | 79 °C |
| Autoignition temperature | 450 °C |
| Lethal dose or concentration | LD50 oral rat 300 mg/kg |
| LD50 (median dose) | LD50 (median dose): 300 mg/kg (oral, rat) |
| NIOSH | MN9625000 |
| PEL (Permissible) | PEL (Permissible Exposure Limit) of Pyrogallol: "5 mg/m3 |
| REL (Recommended) | 5 mg/m³ |
| IDLH (Immediate danger) | 300 mg/m3 |