
Gallic acid has traveled quite a road. Ancient societies spotted its presence in oak galls and bark, not because they were searching for antioxidants, but to solve real problems—like making inks, medicines, and dyes. Over time, chemists started to recognize its chemical backbone, and by the 18th and 19th centuries, practitioners were pulling this compound out of plants with basic tools. Friedrich Wöhler, known for synthesizing urea, proved gallic acid could be produced in a lab, not just foraged from the woods. Back then, finding a stable tannin for ink that wouldn’t fade mattered in practical ways we sometimes forget. In the 20th century, researchers saw gallic acid as more than an artifact. Here, European and Asian scientists published thousands of articles about its structure, its unique phenolic pattern, and its downside—how easily it would oxidize. Innovation did not stop at chemistry. Botanists, pharmacists, and food technologists each found new directions to take these discoveries.
Today, the gallic acid industry looks far different. Manufacturers offer both synthetic and natural grades, depending on the end use. Pharmaceutical firms seek pharmaceutical grade, which means strict purity levels and traceable origin. Food producers often want forms that dissolve easily and mix without clumping. Even with this evolution, one thing still anchors every product: the molecule’s simple ring structure and three hydroxyl groups. One gram in pure powder can fetch a premium, especially when customers demand certificates of analysis and verification of plant origin. Global players aren’t content with the standard form—they push for granular, ultra-fine, and even encapsulated versions, all trying to catch the needs of new customers. Prices jump or drop each year, depending on harvest, synthetic yields, and the surge of new crop diseases in oak and tara trees.
Pure gallic acid presents as pale or off-white crystals, with a mild, sometimes astringent odor. Many who have handled it remember how quickly it colors when exposed to air. That discoloration happens because the phenolic groups oxidize, giving away its free-radical scavenging abilities. The melting point sits just below 265°C, a helpful marker when checking lab samples. It dissolves in water, especially at higher temperatures, and even more readily in ethanol and ether. Some compounders forget: high pH or metall ions can lead to unpredictable reactions, making proper handling key for scale-up. Gallic acid serves as a kind of benchmark for plant phenols—researchers often use it as a standard in assays because of its reliable chemical footprint.
In regulated markets, gallic acid labeling gets serious. Product datasheets need to include CAS number, purity (often above 99% for the pharmaceutical sector), appearance, pH range, and accepted loss on drying. The country of origin, batch number, and storage instructions often run right on the packaging. Heavy metal limits follow international guidelines—typically under a few parts per million. HPLC assays confirm that the material isn’t loaded with similar tannins or unknown residues. For food use, producers must include allergen information, and warn if the product was processed with organic solvents. Some suppliers print QR codes on every drum so that customers can trace their substances from farm to final bag. Each label stands between the customer and a costly recall. Regulatory agencies have no patience for errors—and neither do the multinational buyers.
Extracting gallic acid at scale involves more than boiling bark in water. Smaller, artisanal producers still crush tara pods, infuse with hot water, and then use filtration and acidification to pull out the active compound. Larger facilities leverage enzymatic hydrolysis or fermentation, feeding biomass to specific fungi or bacteria that break down gallotannins into pure acid. Pharmaceutical routes involve synthetic steps: methylation, oxidation, purification with activated charcoal, and then recrystallization. Process engineers face a tough equation—the more pure the yield, the harder the waste is to deal with. Energy costs push up prices, and waste streams need careful handling so plant effluent doesn’t disrupt local ecosystems. Every step can shift the product profile: batch conditions, temperature, and even the glassware matter. This isn’t just chemistry—it’s a constant balance between cost, sustainability, and purity.
Gallic acid rarely stays in its vanilla form for long. In the lab, researchers often esterify it, adding methyl, ethyl, or propyl groups to change its water solubility. Polymeric reactions with formaldehyde produce inks and pigments prized for their resilience. Chemists use gallic acid as a building block, preparing natural antioxidants or conjugating it to drugs so they last longer in the human body. Its carboxylic acid moiety reacts nicely with alcohols, while phenolic hydroxyls chelate metals. That means it binds iron, which explains its ancient use in ink and some of its antioxidant power. Under lab conditions, gallic acid can dimerize or bond with sugars, giving rise to complex glycosides studied for their bioactivity. Each modification shapes its fate—better solubility, slower oxidation, or stronger antimicrobial effect. Tailoring gallic acid for cancer trials or natural pesticide research never follows a single recipe.
Gallic acid has walked under many names: 3,4,5-trihydroxybenzoic acid, tannic acid monomer, and sometimes “China blue” acid in old ink recipes. In the supplement aisle you might spot “oak gall extract” or “tara tannin acid.” Pharmaceutical formularies sometimes list it as “acidum gallicum.” These overlapping names cause real confusion. Product names shift by region—some shipments labeled “gallotannin hydrolysate” may only contain traces of the real compound. In trade, mislabeling can lead to shipment refusals, regulatory fines, and detective work for quality control officers. Anyone buying gallic acid in bulk pays attention to synonyms to avoid hidden costs.
Anyone storing or transporting gallic acid must follow protocol. Skin, eyes, and respiratory tract don’t respond well to heavy exposure, and powder clouds trigger irritation. Facilities invest in extraction systems, full PPE, and elaborate spill procedures. Some manufacturers require workers to log exposure time, keep dedicated clothes, and test for allergens every year. Gallic acid’s reactivity increases with heat and light, so climate-controlled, dry storage solves a lot of headaches. Regulatory agencies want to see solid safety data—worker incident reports, compliant labeling, and emergency plans for accidental releases. Even with strict policies, the human element counts: untrained staff or lax housekeeping will always spell trouble.
The list of uses for gallic acid extends across massive industries. The pharmaceutical world sees it as an antioxidant baseline in supplements, and as a scaffold for anti-inflammatory drugs. Food scientists lean on it as a natural preservative, banking on its power to slow down microbial growth and color changes. Historians know it for its long run as ink and dye mordant. Winemakers once used it to control oxidation in barrels; specialty coffee producers now tout it as an indicator of bean quality. Researchers keep pushing the frontier, combining gallic acid with polymers for medical sensors, using it as a reducing agent to make nanoparticles, or adding it to biodegradable plastics. The demand comes from every corner—cosmetics, textiles, agriculture, even plastics engineering—and industry keeps inventing fresh opportunities.
Research labs all over the world fight to stay ahead, testing new ways to extract, synthesize, and modify gallic acid. Universities run trials to check how derivatives attack tumor cells or block bacterial growth. Multinational companies fund work on non-toxic polymers based on gallic acid frameworks. A regular feature in high-impact journals: comparison of different extraction routes—using ultrasound, microwave assistance, or even green solvents to cut down water and energy use. R&D teams tinker with fermentation conditions, changing microbe strains or feedstocks, hoping to squeeze out extra yield and trim down costs. Competition is intense—no one wants to be left behind in the search for the next big breakthrough, especially as demand for plant-based and sustainable molecules keeps rising.
Toxicity often gets discussed as a footnote, but for gallic acid, it deserves real attention. Animal studies suggest that moderate doses rarely provoke acute toxicity, but chronic exposure points at liver and kidney stress. Case reports from workers exposed to high airborne concentrations flagged respiratory and skin irritation. Regulators look hard at new modifications—any new ester or polymer based on gallic acid demands separate toxicity testing before approval. The compound’s interaction with metals and other dietary ingredients raises questions about possible allergic or mutagenic responses. Researchers in pharmacology focus on dose, duration, and delivery method, hoping to explain the narrow boundary between beneficial antioxidant and unwanted side effects.
The outlook for gallic acid isn’t just bright—it’s wide open. Consumer pressure for natural antioxidants drives companies to seek alternatives to synthetic stabilizers. Food regulations get stricter each year, pushing for compounds with documented safety profiles, and gallic acid fits that need. The field of targeted drug delivery sees promise in gallic acid derivatives that bond with specific tissues or release drugs more slowly. Green chemistry researchers look at ways to turn agricultural waste—husks, pods, even fruit peels—into usable phenolics. Biotechnology startups line up funding to make production scalable without fossil fuels or heavy solvents. All these signals point to an industry that will keep growing, adjusting, and shifting as new technologies and public expectations change the game.
Gallic acid isn’t a buzzword for most people, but this tiny molecule shows up in a lot more places than expected. Found in tea leaves, fruits like blueberries and grapes, and even some tree barks, gallic acid gets plenty of attention for its complex effects on health and industry. My grandmother brewed wild berry teas every winter, claiming they “cleaned the blood.” Back then I chalked it up to old-world wisdom. Turns out, science backs up some of those claims.
Researchers have been studying gallic acid for decades. It has a knack for blocking the kind of cell changes that drive chronic inflammation, and tests on mice and cells point to antioxidant effects as strong as the best-known vitamins. Gallic acid can zap free radicals—the troublemakers behind aging and many diseases. Nutritionists believe getting it through fruits, teas, and even wine could help keep the body’s defense systems in shape.
Cancer researchers have taken notice too. Some lab studies show gallic acid can slow or even stop the growth of certain cancer cells by pushing them into self-destruct mode. More research on humans will tell us if it has a place in treatment, but those early signs hint at real value.
Open up a packaged sauce or a fruit bar, and there’s a chance it stayed fresh thanks to gallic acid. Food producers look for natural ways to stop spoilage and cut down on synthetic preservatives. With its ability to block bacterial growth and keep fats from turning rancid, gallic acid offers a plant-based ingredient that addresses both safety and demand for “clean” labels. The World Health Organization considered it safe for food use, and in my pantry, I trust foods preserved by simple plant extracts more than hard-to-pronounce chemicals.
Beauty aisles now boast anti-aging serums powered by natural antioxidants, and gallic acid gets a spot on that shelf. Creams with this compound claim to fight off sun damage and even out skin tone. Dermatologists see some potential—especially since oxidative stress plays a big part in wrinkles and pigmentation—as long as formulas deliver enough of the active molecule.
Gallic acid also plays a quiet role in technology. If you’ve seen historical documents with brown ink, you’ve seen gallic acid at work—iron gall ink once dominated official papers and artists’ sketches. Scientists still use its chemical reactivity today, whether it’s making advanced coatings for electronics, prepping samples in drug tests, or extracting metals in greener ways. Its versatility reminds me that plant molecules often outperform what human factories can copy.
The more we learn about gallic acid, the more it looks like a bridge between nature and innovation. Sourcing it responsibly matters, though. Over-harvesting of oak galls or other wild plants threatens natural habitats. Modern production methods using fermentation or byproducts from food processing seem like better routes. If industry continues tapping into renewable resources and keeps a close eye on environmental impact, gallic acid will keep delivering benefits—without taking more than necessary from the planet.
Gallic acid shows up in a lot of things I run into—black tea, berries, grapes, and even wine. Fans of herbal supplements see it as a natural antioxidant, and food makers appreciate that it comes from plants and brings flavor, color, and shelf-life benefits. Since some folks raise questions about the safety of almost anything added to food, the spotlight sometimes lands on gallic acid.
The science so far offers plenty of reassurance. Extensive studies from reputable sources, including the U.S. Food and Drug Administration and European Food Safety Authority, have reviewed experiments and human data. Researchers say that the kinds of foods that contain gallic acid—tea, nuts, blueberries—look safe for most people, even in decent amounts.
Studies in animals often examine giant doses, sometimes far above what anyone would eat in a normal diet. Researchers haven’t seen dramatic negative effects at reasonable amounts, either in animal experiments or in studies with humans. In fact, human volunteers have consumed gallic acid as part of their regular diet or as a supplement, and reports of trouble look pretty rare. Its long history in traditional diets and herbal medicine adds more weight to the safety picture.
People with certain rare allergies or metabolic issues might react poorly, but this holds for nearly any food compound. Claims linking gallic acid to cancer or DNA damage show up in old research at very high doses, often far beyond what food or supplements supply. Every scientific panel that’s reviewed these claims says these results don’t apply to eating run-of-the-mill foods or drinking tea.
Antioxidants catch a lot of hype, and gallic acid falls into this camp. Some studies suggest it helps protect our cells from stress and damage. In the lab, it’s shown potential against inflammation, aging, and some chronic diseases. Most research here looks at higher concentrations or cell models, but the fact it’s in so many plant foods links it with better diet quality.
One reason dietitians and doctors talk about eating more plant-based foods comes from finding that the natural compounds like gallic acid support wellness. It’s not a magic bullet, but it’s one of those nutrients connected to why fruits and vegetables give us long-term health benefits.
Supplements throw an extra wrench into the story. People sometimes take big doses without knowing if their body handles megadoses differently than the smaller amounts from tea or berries. With supplements still loosely regulated, you don’t always know the exact dose or purity. Sticking to foods rich in gallic acid, instead of grabbing a high-concentration pill, usually sidesteps these risks.
If you’re not sure about a supplement or you’re taking medication, checking in with a doctor or dietitian helps avoid unwanted surprises. Most folks can enjoy foods with gallic acid as part of a balanced diet. With common sense—choosing whole foods, keeping supplements in check, asking questions—gallic acid poses about as much risk as most other everyday nutrition choices.
References:Gallic acid pops up in all kinds of berries, teas, walnuts, and even red wine. Most folks eat it without giving it a second thought. News around plant compounds tends to use big words, but gallic acid delivers real, down-to-earth value. Doctors and nutritionists have pointed toward its power for years, yet few people talk about it outside science circles.
Every cell gets hit by free radicals daily—from sunlight, pollution, even fried foods. This daily dose leads to oxidative stress, which forms the soil for problems like aging, diabetes, or even cancer. Gallic acid stepped into the spotlight because it acts as a shield against these unstable molecules. More than a thousand studies prove it neutralizes free radicals. It does this more potently than some vitamins, helping cells recover from stress. In real life, that means healthier skin, stronger organs, and a system less likely to break down over time.
Anyone who deals with achy joints after a hike or finds their hands stiff in winter understands inflammation on a personal level. Gallic acid has the knack for calming these flares in the body. Animal studies show less swelling and less pain when gallic acid enters the picture. It may even slow the rate at which inflammation damages healthy tissue. For someone aiming to keep moving into their golden years, this kind of support matters. Most anti-inflammatory drugs come with side effects over time; plant-driven solutions like gallic acid provide a gentle hand.
Heart attacks or high blood pressure don’t start overnight. They creep up, built on years of wear and tear. New research from nutrition journals finds that gallic acid keeps the cardiovascular system calm. Blood vessels stay more flexible, cholesterol levels stay balanced, and blood flows more smoothly. It doesn’t stop there. There’s promising evidence that this acid supports the liver, guards the kidneys, and even supports cells in the gut, where new science says much of the immune response begins.
Supplements crowd health store shelves, but most nutritionists still vote for real food. Gallic acid packs itself into blueberries, strawberries, grapes, black tea, and walnuts. For many, it’s just about choosing a handful of berries or switching up breakfast tea. My own family swapped soda for hibiscus tea—less sugar, more gallic acid. I’ve seen teen acne flare-ups ease, and our skin looks brighter after a few months. Small steps still count.
Doctors look at consistent patterns, not just hype. The Mayo Clinic, Cleveland Clinic, and Harvard School of Public Health all direct people toward plant-rich diets partly for these polyphenols and acids. Ongoing clinical trials study how gallic acid may partner with cancer treatments, or how it might help diabetics manage blood sugar. These are hard claims, built on thousands of patient stories and years of observation.
No compound works like magic. Some people feel digestive upset with too much tea or berries. Balance remains key. Diversity in the diet—nuts, fruits, teas—means the body draws from a bigger toolbox. If someone faces chronic illness, a quick chat with a doctor about adding more gallic acid-rich foods, or even supplements, makes sense.
A lot of folks see gallic acid’s name listed on tea analyses or supplement ingredient decks and shrug. But this natural polyphenol, found in things like oak bark and green tea, comes with a set of quirks that mean it won’t last forever if handled like table salt. I spent a year working in a chemistry lab at a university, stacking all kinds of reagents in tightly-packed cupboards. Storage, even for compounds that barely see the limelight, decides whether experiments go right or wrong. Gallic acid’s not the fussiest compound around, but don’t trust it to keep itself pure if treated carelessly.
The structure of gallic acid, with those hydroxyl groups, lures in water from the air. Open the container on a muggy day, and the powder clumps. Give it enough time in humidity, and some of its antioxidant punch fizzles out. Plus, even regular fluorescent bulbs turn into troublemakers. Ultraviolet from lights or sunlight breaks gallic acid down. I’ve seen open bottles go yellowish over time. That’s a sign trouble started brewing.
A half-closed plastic bag is an invitation for spoilage. At the lab, glass bottles with rubber gasket lids work best, but a tightly twisted screw cap on a plastic bottle stands up well for routine use. For anyone who needs gallic acid to do double duty over months—whether for food testing, supplementation, or dye experiments—keep desiccants inside the storage vessel. Silica gel packs, easily reused after drying in an oven, pull extra water from the air.
Heat stokes up the breakdown rate. It also changes the flavor if someone’s adding gallic acid to foods. A shelf in a dry storage room stays cool enough, but for long-term stashing, the refrigerator works wonders. Freezer storage complicates things, as pulling the bottle in and out draws condensation. Still, if you’re dealing in massive bulk, low temperatures mean protection.
Gallic acid doesn’t have to be a mystery guest in the storage closet. Label every container with the opening date. In one research group, we started writing use-by dates six months out from first open. Sure, the compound keeps longer than that, but strict dating made it clear which bottle to tap first and kept everyone honest about tossing the old stuff.
Some overlook the risk of storing gallic acid near strong-smelling or corrosive chemicals. Uncapped bleach or ammonium hydroxide in the same shelf? Not smart. Even trace amounts of iron or copper in a shared scoop causes the acid to react, forming colored complexes. At my old lab, a caretaker left a steel spatula in one container and got a lovely but useless greenish paste a couple weeks later.
Quality control doesn’t just save dollars; it keeps experiments on track. Low-quality plastic with leaching risks, or warped containers that don’t seal tight, throw off chemical purity and raise waste disposal concerns. Nobody enjoys the surprise of opening a bottle to find a discolored, clumped mess instead of pure, fluffy powder.
Simple habits protect gallic acid’s integrity: airtight, dry, dark, cool, and segregated from reactive substances. For people in labs, kitchens, or supplement warehouses, a little extra care means results stay true, stock lasts longer, and safety issues fade into the rearview. Every scoop matters, especially if the science or product quality hangs in the balance.
Gallic acid pops up all over the place: in tea, berries, nuts, and even some wines. This natural compound gets plenty of attention for its antioxidant punch. After a chat with my grandmother, who still brews a mean blackberry tea, I started reading up on gallic acid long before it made headlines. Researchers credit it for fighting off free radicals, supporting the body’s defense systems, and offering a little extra something for folks worried about aging or inflammation.
Eating fruits and nuts rich in gallic acid hasn’t raised alarms through history. The occasional upset stomach can happen if you overdo it with any food, and gallic acid isn’t an exception. Some folks with sensitive digestion report mild stomach cramps or gas after taking concentrated supplements. I once tried a dietary booster with extra gallic acid and felt a bit bloated—nothing serious but enough to remind me that more isn’t always better.
Gallic acid can lower blood sugar in certain lab studies. People with diabetes, or anyone taking medication for blood sugar control, should be careful. A friend managing type 2 diabetes asked his doctor about high-dose supplements. The advice came quick: stick to food-based sources unless a medical professional gives the go-ahead.
Allergies sometimes spring up, even with natural compounds. It’s rare, but skin rashes, itching, or other signs of sensitivity could indicate a problem. Mixing new foods or supplements with ongoing prescriptions can also produce side effects, and not always the good kind. One study relating to breast cancer cells found that gallic acid can disrupt some kinds of hormonal therapy. Most headlines don’t mention this risk, but it matters to anyone dealing with a serious health issue.
Manufacturers market gallic acid supplements hard. Skipping doctor visits or ignoring personal health history because a bottle promises “antioxidant protection” sets folks up for trouble. In my own circle, self-diagnosing and dosing proves tempting, but these decisions call for professional advice. Foods offer gallic acid in small, manageable amounts that the body can process naturally. Tablets and powders go way past that level.
Researchers keep examining gallic acid’s benefits, and the science continues to shift. Not every study matches real-world experience. The Food and Drug Administration doesn’t closely monitor most supplements, so safety testing lags behind claims. Just because a substance comes from nature doesn’t mean it works for everyone or that it stays safe at any dose.
Doctors and registered dietitians offer the best advice when it comes to adding new compounds like gallic acid to a routine. If new symptoms appear—skin reactions, digestive trouble, sudden changes in energy—stopping supplements and getting professional input matters. Schooling myself on the ingredient list, sticking to recognized sources, and looking up clinical studies offer more protection than trusting flashy claims on social media.
Diet speaks louder than supplements. Fresh fruits, nuts, and teas naturally rich in gallic acid belong in a balanced eating pattern. Supplements may help certain groups, but risks do exist—especially in high doses, or if mixed with prescription medicines. Putting health on autopilot never ends well; staying involved, asking questions, and seeking out trustworthy sources smooths out the road to better living.
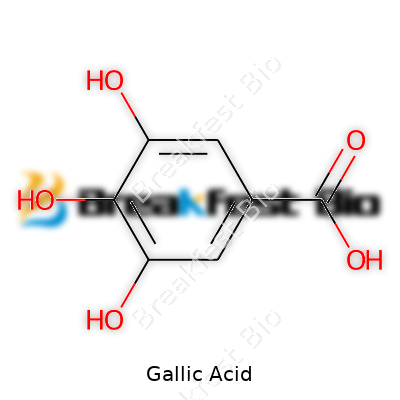
| Names | |
| Preferred IUPAC name | 3,4,5-Trihydroxybenzoic acid |
| Other names |
3,4,5-Trihydroxybenzoic acid
C7H6O5 Gallicin Acide gallique Acidum gallicum Pyrogallol-5-carboxylic acid Benzoic acid, 3,4,5-trihydroxy- Digallic acid |
| Pronunciation | /ˈɡæl.ɪk ˈæs.ɪd/ |
| Identifiers | |
| CAS Number | 149-91-7 |
| Beilstein Reference | 136231 |
| ChEBI | CHEBI:30778 |
| ChEMBL | CHEMBL809 |
| ChemSpider | 1001 |
| DrugBank | DB04272 |
| ECHA InfoCard | 100.007.366 |
| EC Number | 3.1.1.11 |
| Gmelin Reference | 3587 |
| KEGG | C00792 |
| MeSH | D005700 |
| PubChem CID | 370 |
| RTECS number | LW9275000 |
| UNII | 9G2MP84A8V |
| UN number | UN2811 |
| Properties | |
| Chemical formula | C7H6O5 |
| Molar mass | 170.12 g/mol |
| Appearance | White to slightly yellowish crystalline powder |
| Odor | Odorless |
| Density | 1.694 g/cm³ |
| Solubility in water | slightly soluble |
| log P | 0.7 |
| Vapor pressure | 2.74E-8 mmHg at 25°C |
| Acidity (pKa) | 4.0 |
| Basicity (pKb) | 8.37 |
| Refractive index (nD) | 1.725 |
| Dipole moment | 1.83 D |
| Thermochemistry | |
| Std molar entropy (S⦵298) | 181.1 J·mol⁻¹·K⁻¹ |
| Std enthalpy of formation (ΔfH⦵298) | -812.51 kJ/mol |
| Std enthalpy of combustion (ΔcH⦵298) | -1242.0 kJ/mol |
| Pharmacology | |
| ATC code | A01AB11 |
| Hazards | |
| Main hazards | Harmful if swallowed, causes skin and eye irritation |
| GHS labelling | GHS07, GHS06 |
| Pictograms | GHS07 |
| Signal word | Warning |
| Hazard statements | H302: Harmful if swallowed. |
| Precautionary statements | P264, P270, P301+P312, P330 |
| NFPA 704 (fire diamond) | 2-1-1 |
| Autoignition temperature | 400 °C |
| Lethal dose or concentration | LD50 (oral, rat): 5,000 mg/kg |
| LD50 (median dose) | LD50 (median dose): Rat oral 5,000 mg/kg |
| NIOSH | WF8050000 |
| PEL (Permissible) | Not established |
| REL (Recommended) | 30 mg |
| IDLH (Immediate danger) | Not listed |
| Related compounds | |
| Related compounds |
Tannic acid
Pyrogallol Ellagic acid Methyl gallate Propyl gallate |