
The journey of 3,4,5-Trimethoxybenzaldehyde traces back over a century, with references in early synthetic organic chemistry literature as researchers explored structural variations of vanillin and other aldehydes for fragrance development and medicinal chemistry. Early labs worked with crude techniques, but perseverance led chemists like Perkin and Baeyer to recognize the unique reactivity created by placing methoxy groups on the benzene ring. The product's role expanded quickly as downstream industries picked up on its potential—not just for dyes or scents, but as a scaffold for new pharmaceuticals and specialty chemicals. Companies and academic labs alike kept circling back, refining routes and boosting purity. Even today, this compound shows up in retrospectives or patent searches, connecting generations of innovation.
3,4,5-Trimethoxybenzaldehyde carries weight thanks to its distinct aromatic character and reactivity. Found as a crystalline solid, it stands out by offering three methoxy groups circling an aldehyde core, unlocking options for transformations. Labs and manufacturers rely on it as a key intermediate in synthetic routes for drugs, natural product analogues, and performance chemicals. Scale ranges from grams in R&D settings to tens of kilos for production, feeding into industries from pharma to dyes. Its odor and behavior signal the sharp presence of the aldehyde group, yet the methoxy groups ease handling compared to other aromatic aldehydes, making it a preferred choice in many recipes and a staple in storerooms for those who value versatility.
Showing up as off-white to pale yellow crystals, 3,4,5-Trimethoxybenzaldehyde offers impressive solubility in alcohols, ethers, and polar solvents thanks to its multiple methoxy groups. The substance melts at about 75–78°C and boils in the 205–210°C range under reduced pressure, so it survives most mild heating in labs or reactors. The structure—three methoxy substituents connected to a benzene ring with the fourth position taken by an aldehyde—shows how basic chemical intuition, with a glance at electron-donating or withdrawing effects, can predict reactivity patterns. The methoxy clusters shield the aromatic core, allowing selective reactions on the aldehyde handle while stabilizing the ring from harsh oxidation or substitution. Such a profile sets it apart from simpler benzaldehydes, both in ease of purification and in flavor for multistep synthesis, since the groups can both steer and buffer chemical change.
Producers market this material with clear specifications. Pure product meets a GC or HPLC purity of at least 98%, sometimes stretching to 99.5% for tougher applications. Labs check for color, melting point, and residual solvents. Moisture content matters for hydrolysis-prone reactions, so standard labeling spells out storage advice—cool, dry, with sealed containers and dark glass. Proper CAS and EC numbers, hazard phrases, and transport labels round out the label, keeping the supply chain aboveboard. Details such as batch number, date of manufacture, and recommended disposal steps speak to regulations and conscientious handling, helping companies track every gram from loading dock to bench or pilot plant. Such specificity keeps both the material and its users in the clear.
Most 3,4,5-Trimethoxybenzaldehyde gets synthesized by oxidizing 3,4,5-trimethoxytoluene or by selectively formylating trimethoxybenzene using robust reagents. Industrial setups lean on careful control of reaction temperature, catalyst concentration, and time—any shortcut and the mix will host side products or incomplete conversion. Classical methods—like the Reimer-Tiemann formylation using chloroform and alkali—gave researchers the template, but environmental concerns and worker safety have sparked adopters of milder, greener techniques. On a small scale, chemists enjoy Swern oxidation or Vilsmeier–Haack approaches, using reagents that keep methoxy groups untouched yet boost the aldehyde signal. After reaction, purification by recrystallization or distillation yields stable material ready for the next step. It’s clear that each method leaves its fingerprint, influencing cost, scale, and product profile.
The aldehyde group in 3,4,5-Trimethoxybenzaldehyde invites all sorts of organic chemistry play: reductive amination, condensation for forming hydrazones or oximes, and Wittig reactions for building new carbon-carbon bonds. The methoxy decorations resist nucleophilic attack and block electrophilic substitution at their respective positions, channeling additions or substitutions toward the aldehyde or the remaining positions on the aromatic core. Many researchers have coupled it with malonates, enamines, or aryl halides for advanced pharmaceutical intermediates. Protection and deprotection strategies around the methoxy or aldehyde extend its utility even further. Chemists tailor modifications based on intended end product—moving beyond simple benzaldehyde as a building block, leveraging the stability and unique electron profile for innovative applications.
Through global trade, this chemical answers to several names: 3,4,5-trimethoxybenzenecarbaldehyde, 3,4,5-TMBA, and even its Registry Number (CAS: 86-81-7). Some catalogs call it benzaldehyde, 3,4,5-trimethoxy-, while pharma and research labs sometimes list it as “trimethoxy aromatic aldehyde” for clarity amidst inventory. While synonyms help source from multiple suppliers, real-world users recognize the compound by sight—solid granules in brown-tinted bottles with labels that echo these variations. Labeling standards demand clarity to avoid mix-ups, so each shipment logs relevant regulatory phrases, synonyms, and manufacturing details worthy of a tidy record book.
Working safely with 3,4,5-Trimethoxybenzaldehyde doesn’t mean just gloves and goggles. The aldehyde can irritate skin and eyes, and inhalation may trigger headaches or slight dizziness for sensitive users, as flagged in MSDS documents worldwide. Strict inventory control and proper storage—no sunlight, tight lids, temperature below 25°C—reduce spoilage and exposure. Spills need prompt cleanup with inert absorbents; good ventilation or fume hoods limit vapors. Waste streams get routed through special disposal channels to prevent environmental harm. Regulatory frameworks like OSHA, REACH, and GHS keep handlers attentive, with hazard labels and training forming a daily routine. Lesson learned after years in the lab: prevention and good habits beat cleanups and paperwork any day.
Drug makers and researchers have found a home for 3,4,5-Trimethoxybenzaldehyde in the construction of active pharmaceutical ingredients. Historically famous as a precursor for mescaline and related psychoactive compounds, it shifts in today’s landscape toward legitimate blocks for antihypertensives, antivirals, or neuromodulators. Dye manufacturers tap into its electron-rich ring for novel pigments, offering stability and vibrancy. In the fragrance sector, it gives rise to musky or woody notes prized for their unique character. Its crossing of boundaries—being picked for both specialty chemicals and mainstream intermediates—shows how diverse the demand runs. For those engineering new molecules, it’s not just a source for classic reactions, but a springboard for creativity, which is hard to overstate.
Cutting-edge labs pour resources into exploring new routes to trimethoxybenzaldehyde derivatives, with published papers and patents chronicling fresh transformations nearly every quarter. The focus leans toward greener synthesis, using milder oxidizing agents or recyclable catalysts, as both companies and universities answer societal pushes for clean chemistry. On the application side, teams probe deeper into the pharmacological activity of ring-substituted analogues, aiming for better efficacy and safety in therapeutic leads. At the same time, material scientists look for improved dyes or polymers that draw on the core benzaldehyde structure. Funding cycles and reviewer panels probe for novelty, but real advancement comes from researchers who know the quirks of this molecule—its stubborn stability and its flashes of reactivity.
Years of animal and in vitro assays reveal that 3,4,5-Trimethoxybenzaldehyde carries lower acute toxicity than many other aromatic aldehydes, thanks to the electron-donating effects of its methoxy substituents. Still, studies document skin sensitization or mild irritation, reminding manufacturers and users that caution outlasts assumptions. Chronic exposure data remain sparse for the general population, but regulatory bodies urge vigilance, especially in pharmaceutical settings where metabolites could interact unpredictably. Newer reviews stress the need for close monitoring in workers, encouraging blood tests or airborne sampling in industrial environments. Industry-wide adoption of green chemistry and improved PPE speak to a culture recognizing both risk and responsibility.
Rising demand for safer and more selective pharmaceutical ingredients keeps the future of 3,4,5-Trimethoxybenzaldehyde bright. Start-ups and established chemical producers alike go after more sustainable synthesis—not simply for regulatory compliance, but for genuine savings in energy and waste. Genetic screening and AI-driven molecular design predict continued value for trimethoxy-substituted cores in drug discovery pipelines. In material science, designers eye this molecule’s structure for next-generation dyes and organic electronics, where the unique substitution pattern hints at new optical and electronic properties. The intersection of tradition and innovation will likely keep this compound buzzing in research journals, patent applications, and behind the scenes on manufacturing floors, supporting work that rarely reaches headlines yet shapes everything from new medicines to the colors on our walls and screens.
3,4,5-Trimethoxybenzaldehyde has become a talking point in labs and research circles. This chemical, often recognized by its white to off-white crystalline appearance, pops up in synthetic pathways for pharmacological compounds, fragrances, and advanced materials. Most bottles shipped for research purposes sport a purity of at least 97% or 98%. Top-shelf options reach 99% or a little higher, and some sources claim even finer grades for analytical needs.
High purity means minimal contamination, and that's not a detail worth glossing over. Any chemist who has struggled with stubborn byproducts during synthesis or faced yield drops can relate. Impurities act like uninvited guests—they can change how a reaction flows, introduce false signals during analytical work, and even threaten safety if left unchecked. The tiny percentage points matter, especially in pharmaceutical research, where stray molecules sometimes mimic or block a drug’s effect.
The number listed on a bottle results from rigorous testing. Labs check samples using HPLC, GC-MS, and similar methods. Reputable suppliers, those committed to trusted science, back up their claims with certificates of analysis. Reading these reports often feels like detective work. Chromatograms plot the chemical truth. Peaks show what’s present in the bottle; missing or jagged peaks point to something off.
It’s tempting to assume a few tenths of a percent won’t matter, and sometimes, in very basic experiments, that’s true. But most downstream work won’t forgive laziness. Small synthesis errors snowball. Work with a 95% grade to save money, and you might spend days filtering out side products or redoing runs. Early in my days at a small materials startup, we once grabbed a cheaper, lower grade for a pilot project. The end result: odd colors, bad yields, and the embarrassment of scrapping an entire batch of product. Lesson learned.
Impurities in 3,4,5-Trimethoxybenzaldehyde don’t always just lower yields; they can directly alter results in biological screening, skew melting points, or introduce hidden hazards. Some manufacturing batches may contain trace solvent residues, leftover reagents from the synthesis, or even unintended positional isomers. For pharmaceuticals, even tiny slips trigger regulatory alarms.
Anyone sourcing this compound should look beyond the catalog. Ask for batch certificates, grill suppliers about their purification processes, and request third-party verification if the stakes are high. Labs that rely on assumptions often lose both time and credibility.
Reproducible science depends on trust in every bottle. Investing in high-purity material almost always repays itself during downstream synthesis, framing cleaner spectra, and supporting safer, more reliable datasets. High-purity 3,4,5-Trimethoxybenzaldehyde isn’t just an expense—it’s a foundation for results you won’t end up questioning days or months down the line.
Demand for transparency from suppliers keeps everyone on their toes. Researchers need to hold each other accountable, share details on problematic batches, and push for better documentation. The simple act of requesting that extra certificate can sometimes unearth corners suppliers tried to cut. Over years in the lab, I’ve seen high-purity reagents pay off in faster, more effortless synthesis and more confident publication. This chemical is no exception.
Working with organic compounds in a lab gives a close look at why chemists reach for 3,4,5-Trimethoxybenzaldehyde. This compound finds a central spot in pharmaceutical research. Take the synthesis of trimethoxy derivatives—3,4,5-Trimethoxybenzaldehyde often acts as a starting block for key medicines. Galantamine, a treatment for Alzheimer’s disease, gets its core structure from reactions involving this aromatic aldehyde. The journey from benchtop to bedside usually has a few hurdles, so high-purity starting materials make a difference in research yields and reliability.
Vibrant colors in the textile and paint industries often start in chemistry labs. This compound serves as a critical intermediary for making synthetic dyes. Because it mixes easily with other aromatic rings, 3,4,5-Trimethoxybenzaldehyde can help tune the final color and stability of many pigments. Textile manufacturers and artists hunting for novelty colors benefit from such custom approaches. I’ve seen fabric designers specify this aldehyde for unusual hues or to boost colorfastness in demanding conditions.
Inside the world of perfumes, creating new scents demands molecules that can bridge floral, spicy, or woody notes. 3,4,5-Trimethoxybenzaldehyde appears in several aromatic compositions. Its structure lends a soft, almond-like undertone—one reason perfumers value it when seeking sophisticated blends. Sometimes, synthetic fragrances draw inspiration from nature, but rely on this compound for batch consistency. Quality control is easier when starting from a reproducible chemical scaffold like this one. Years ago, smelling esters derived from this compound brought out subtle differences in each batch, showing how the source materials influence the end result.
Few chemicals play such a flexible part in synthetic organic chemistry. In advanced reactions, the three methoxy groups on the aromatic ring make 3,4,5-Trimethoxybenzaldehyde an excellent partner for condensation and coupling work. Chemists use it to build frameworks for more complicated compounds, some destined for the medicine cabinet, others for research tools. It’s reliable and easy to handle, which helps streamline work for graduate students and career chemists alike. Personally, I’ve used it for synthesizing reference standards for analytical labs, where reliability and traceability in origin really count.
Industrial and academic labs need to keep an eye on safety and environmental issues as demand for intermediates rises. Safe handling practices, regular air monitoring, and using proper ventilation matter, especially as regulations grow stricter worldwide. Open collaborations with suppliers encourage higher transparency on synthetic routes and contaminant profiles. Switching to greener chemistry, such as solvent-free reactions or catalytic methods, helps labs shrink their footprint. From my perspective, the real progress appears once a team embraces life-cycle thinking for these materials, from raw ingredient selection to final product disposal.
Reliable sourcing counts. Reagent quality can influence research timelines, project budgets, and even regulatory paperwork. It’s no longer enough to grab the cheapest option. Checking certificates of analysis, understanding manufacturing practices, and seeking suppliers who value quality keep research labs on solid footing. I’ve seen teams hit project delays because of inconsistent batches, showing the real-world importance of investing in quality sourcing for every critical reagent, including 3,4,5-Trimethoxybenzaldehyde.
I’ve worked in labs that run on chemicals like 3,4,5-Trimethoxybenzaldehyde. Every year, I see the same issue: people treat storage as an afterthought. That’s a problem. Mishandling even simple-looking substances can mean spoiled research, wasted money, or worse—risk to health. This compound, an off-white powder with a pleasant enough look, has some bite. Anyone building a stable lab knows that safety and purity start with where and how you put your bottles and jars.
Once, I watched a coworker stash a bag near a sunny window. That batch went yellow in a month. We all learned something: light does more damage than people expect. Sunlight nudges this chemical, breaking it down bit by bit. Keeping it in a brown glass bottle shields it from direct and indirect light, stopping degradation before it starts. Brown bottles aren’t marketing hype—they’re lab basics with proven results.
A dry place makes just as much sense. Humidity gives organic powders trouble. Over time, moisture creeps inside regular plastic lids. It can clump powders, spark slow decomposition, or form harmful byproducts. After losing a batch to clumping, I switched all relevant chemicals to airtight glass jars with rubber seals. No more mysterious reactions, and shelf life jumped. Simple tweaks like silica gel packets in the cabinet absorb humidity and cost almost nothing.
Room temperature means something different to everyone. In summer, some stockrooms climb above 30°C. At these temperatures, chemicals like 3,4,5-Trimethoxybenzaldehyde see an uptick in unwanted reactions. Consistent coolness keeps chemicals stable. Refrigerators set between 2–8°C work well, but frost builds up in old fridges can trap bottles. I’ve found purpose-built flammable cabinets with built-in ventilation nail the right balance, staying steady without freezing.
Powders scatter easily. Leaving containers open, even for minutes, might let dust or moisture in. Once, I ruined weeks’ worth of material because of a badly seated cap. After that, closing each container tightly right after use became a habit, not a chore. Labels wear off, so adding a backup label or clear tape keeps things readable. Writing open dates and initials on bottles gives clear reminders—no one debates freshness or provenance. Rotating stock helps too. Using older stuff first means product gets less time to spoil.
Years of experience taught me: it always costs less to prevent loss than to deal with spoiled materials. Keeping 3,4,5-Trimethoxybenzaldehyde in airtight, lightproof bottles, tucked away from heat and humidity, makes all the difference. Clean hands, clear labels, and a habit of double-checking closures prevent most problems. Take small, effective steps—they offer peace of mind and make long-term work safer and more productive.
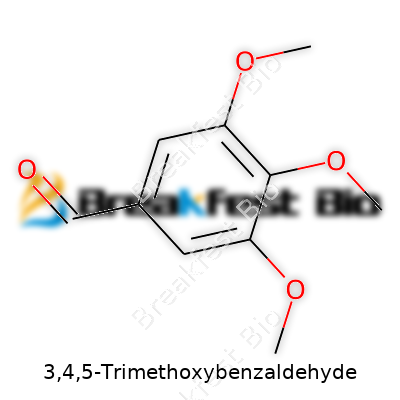
3,4,5-Trimethoxybenzaldehyde goes by the molecular formula C10H12O4. Its structure features a benzene ring with methoxy groups at positions 3, 4, and 5, plus an aldehyde group attached to the carbon ring. The molecular weight comes in at approximately 196.20 grams per mole. These numbers offer more than a formula—they point to what this compound can do.
Scanning a list of aromatic aldehydes, 3,4,5-trimethoxybenzaldehyde pops up frequently in syntheses tied to pharmaceuticals and specialty chemicals. Its backbone plays a part in making certain antihypertensive drugs and some derivatives lead to anti-cancer candidates. Being able to jot down “C10H12O4” isn’t just for lab notes; it sets up a chemist to figure out how to manipulate the molecule for something bigger. I remember visiting a university lab where graduate students started with this precise aldehyde, walking through each transformation, watching as the methyl groups steered reactivity one way or another.
Precision matters. Even small errors in the molecular formula or molar mass can ripple through research projects. One year, a formulation error traced back to a single methoxy misplacement threw off the yields for a whole set of candidate molecules. It left everyone scrambling for better quality checks—and a renewed respect for the raw data.
Labs need solid sourcing and logic to verify identities and avoid those costly mistakes. Reliable suppliers and certificates of analysis matter. Once, a batch from an unfamiliar vendor brought frustrations: the product was off by a few grams, testing didn’t match, and synthesis plans paused for days. That incident underscored a simple truth: quality control for even these base ingredients shapes the outcome downstream.
Analytical standards like HPLC, NMR, and mass spectrometry are not overkill here. Clear signals for all protons—especially those on the methoxy and aldehyde groups—let technicians move forward confidently. Chemistry never rewards shortcuts.
To boost reliability, standard references provided by governmental sources or trusted chemical catalogs reduce risk. Up-to-date analytical methods keep identity checks honest and quick, saving time and money for everybody involved. On the education side, teaching students to cross-check formulas with experimental spectrum readings helps the next generation avoid routine slip-ups.
In industrial settings, regular audits of suppliers and well-maintained documentation systems keep mistakes out of the pipeline. I’ve seen organizations run tight verification steps, pairing chemical barcoding with automated checks, which helps flag inconsistencies before they reach the reaction flask. For the rest of us, patience and a little skepticism—cross-referencing, re-calculating—pay off far more than rushing through details.
C10H12O4 does not exist in a vacuum. Getting its formula and weight right means opening doors for useful science, improved medicines, and safer processes. Small details, handled with care, set a strong foundation for progress in chemistry and beyond.
Those of us who have spent time in a lab can spot patterns: certain chemicals turn handling into a risky dance, while others let us get on with business as usual. 3,4,5-Trimethoxybenzaldehyde pops up fairly often, especially in settings tied to pharmaceuticals and research. It looks unassuming—a crystalline solid, faintly yellow. Yet don’t judge by looks alone.
Contact with this compound isn’t harmless. Get it on your skin—it can trigger irritation or a rash. Breathe in dust from mishandling, and you risk coughing, sneezing, or a scratchy throat. Accidentally swallow any amount, and nausea or stomach pain may follow. I’ve seen a few rookie chemists brush off gloves after a spill, thinking washing hands later will take care of things. Red patches and itching that last for hours usually make a stronger case for wearing proper protection next time.
Chronic exposure is less studied. We don’t have pages of data laying out long-term biases, but that uncertainty shouldn’t lull anyone into a shrug. Years spent around solvents and fine organics taught me to respect even “mild” reagents. It’s not only direct effects—we think about what happens if similar exposures add up across a week, not just a single shift.
Some compounds threaten dramatic burns or explosions. 3,4,5-Trimethoxybenzaldehyde isn’t particularly volatile, yet that lulls some folks into careless storage. It can still smolder if tossed into a fire, and burning organic solids release dense, toxic smoke. Everyone in my old academic department learned early to stash organics in tight-sealing containers, away from flames or heaters. Moisture and sunlight speed up breakdown, so stick this chemical somewhere cool and dark.
Simple discipline—isolating flammable items, marking bottles clearly, keeping an updated inventory—prevents headaches and emergencies. A few seconds spent double-checking tags or sorting cabinets pays off every year.
Lab coats and goggles matter here. Anyone working with powders—including 3,4,5-Trimethoxybenzaldehyde—uses gloves and keeps face shields nearby. Ventilation isn’t just a rule for mixing solvents. Small organics generate dust that lingers; local hoods or bench fans keep particles from drifting into lungs or landing on your sandwich at lunch.
Clean-up plans should be plain and rehearsed. Sweeping up powders works only after the surface is dampened down. Household cleaning wipes can spread fine particulates, so we hit contaminated spots with appropriate solvents then dispose of rags professionally. In shared spaces, labeling hazards with clear stickers or posters keeps everyone on the same page.
Most labs encourage buddy systems. Having an extra set of eyes helps spot risks or mistakes—two pairs of gloves make for lighter work and safer practice.
Chemical disposal often receives less attention than splashy mishaps, but neglect here adds up. Pouring leftovers down a sink taints water supplies. These aldehydes need special collection and treatment, routed through official hazardous waste channels. Every institution I’ve worked in sets weekly pickups and clear bins for organic waste. For solo researchers or small shops, local councils or universities offer advice or drop-in centers.
3,4,5-Trimethoxybenzaldehyde doesn’t command headlines like some deadly reactives. Still, real safety comes from small habits: wearing the right kit, telling colleagues about a spill, locking chemicals away before distractions pull you from the bench. Staying curious about every reagent we touch protects not just one person, but everyone who works nearby. That respect for what lands in our flasks and jars stays with us—long after individual experiments fade from memory.

| Names | |
| Preferred IUPAC name | 3,4,5-Trimethoxybenzaldehyde |
| Other names |
3,4,5-Trimethoxybenzenecarbaldehyde
Trimethoxybenzaldehyde 3,4,5-TMBA Benzenecarboxaldehyde, 3,4,5-trimethoxy- 3,4,5-Trimethoxy benzaldehyde |
| Pronunciation | /ˌtraɪˌmɛθ.ɒk.siˌbɛn.zælˈdɛɪd/ |
| Identifiers | |
| CAS Number | 86-81-7 |
| 3D model (JSmol) | `3D66B0A0EEGCAFQFAIBGCKB` |
| Beilstein Reference | 1448982 |
| ChEBI | CHEBI:28310 |
| ChEMBL | CHEMBL20372 |
| ChemSpider | 11400 |
| DrugBank | DB04163 |
| ECHA InfoCard | 03bdbb04-cf8a-4dbb-b599-6cfbf6b0d857 |
| EC Number | 206-254-7 |
| Gmelin Reference | 71438 |
| KEGG | C08361 |
| MeSH | D015761 |
| PubChem CID | 70018 |
| RTECS number | DB5750000 |
| UNII | V8C8R0F73T |
| UN number | 2811 |
| CompTox Dashboard (EPA) | DTXSID7035223 |
| Properties | |
| Chemical formula | C10H12O4 |
| Molar mass | 196.19 g/mol |
| Appearance | White to off-white crystalline powder |
| Odor | Odorless |
| Density | 1.18 g/cm³ |
| Solubility in water | Slightly soluble |
| log P | 1.89 |
| Vapor pressure | 2.5E-4 mmHg (25°C) |
| Acidity (pKa) | 7.2 |
| Basicity (pKb) | -3.0 |
| Magnetic susceptibility (χ) | -60.7·10⁻⁶ cm³/mol |
| Refractive index (nD) | 1.577 |
| Viscosity | 0.8585 cP (25°C) |
| Dipole moment | 3.13 D |
| Thermochemistry | |
| Std molar entropy (S⦵298) | 364.6 J·mol⁻¹·K⁻¹ |
| Std enthalpy of formation (ΔfH⦵298) | -262.6 kJ/mol |
| Std enthalpy of combustion (ΔcH⦵298) | -1643.3 kJ/mol |
| Pharmacology | |
| ATC code | Not assigned |
| Hazards | |
| Main hazards | Harmful if swallowed. Causes skin and eye irritation. May cause respiratory irritation. |
| GHS labelling | GHS07, Warning |
| Pictograms | GHS07 |
| Signal word | Warning |
| Hazard statements | Hazard statements: H302-H315-H319-H335 |
| Precautionary statements | P261, P280, P304+P340, P312, P405, P501 |
| Flash point | > 157°C |
| Autoignition temperature | Autoignition temperature: 540°C |
| Lethal dose or concentration | LD50 (oral, rat): 1890 mg/kg |
| LD50 (median dose) | LD50 (median dose) of 3,4,5-Trimethoxybenzaldehyde: **1,720 mg/kg (rat, oral)** |
| NIOSH | VX8575000 |
| PEL (Permissible) | Not established |
| REL (Recommended) | 10 mg/m³ |
| Related compounds | |
| Related compounds |
Vanillin
Syringaldehyde 3,4,5-Trimethoxybenzoic acid Gallic acid 3,4,5-Trimethoxytoluene 3,4,5-Trimethoxybenzyl alcohol |