
Curiosity in benzoic acid derivatives grew stronger in the early twentieth century, driven by innovations in organic synthesis. Chemists, digging into coal tar fractions and natural plant extracts, stumbled upon new aromatic acids with unfamiliar functional groups. 3,4,5-Trimethoxybenzoic acid began to surface as researchers explored methylation reactions. In the 1940s and 1950s, pharmaceutical labs, motivated by the search for improved therapeutics, deepened their focus on methoxy-substituted aromatic acids. Scientists found that the trimethoxy configuration lent itself well to both natural compound analogues and new medicinal chemistry leads. By the 1970s, journals published stepwise synthesis methods and biological evaluations, setting the stage for broader commercial interest. Recognizing value beyond synthetic benchwork, companies refined production and characterization, shifting 3,4,5-trimethoxybenzoic acid from a niche compound into a mainstay for further chemical innovation.
This aromatic acid stands out with three methoxy groups attached to the benzene ring, specifically at 3, 4, and 5 positions. These modifications tweak how the acid participates in chemical reactions, making it valuable in both academic and industrial R&D. Researchers prize the molecule as a scenery for new chemical transformations, thanks in part to a stable structure and predictable reactivity. Vendors keep it stocked as a crystalline solid, offering quantities that range from a few grams in a research lab to bulk supplies for industrial users. High demand continues from those who seek building blocks for advanced materials, pharmaceutical intermediates, and even plant-based chemical studies. Clients care about the balance between purity, availability, and price, putting pressure on suppliers to keep improving their production and quality assurance methods.
The pure form of 3,4,5-trimethoxybenzoic acid appears as white to off-white crystals. Users report a melting point in the range of 180–184°C, which points to a solid that resists decomposition under typical lab conditions. The molecular formula C10H12O5 and a molar mass near 212.2 g/mol make calculations straightforward for synthetic chemists. Its methoxy substitutions give it low water solubility, but alcohols and organic solvents like acetone or chloroform dissolve it readily. This compound exhibits moderate acidity, with the carboxylic acid group remaining reactive for esterification, amidation, or coupling chemistry. Its high level of aromatic substitution means its electron distribution makes it less likely to oxidize compared to unsubstituted benzoic acids. For developers of chemical processes, these traits mean dependable handling and predictable behavior under common reaction conditions.
Vendors who care about reputations offer 3,4,5-trimethoxybenzoic acid at purities exceeding 98%, often tested by HPLC, NMR, and melting point analysis. Bottles arrive labeled with clear batch numbers, production dates, and hazard classifications. Many firms provide certificates of analysis that document spectral data and impurity profiles alongside the expected CAS number (86-81-7). Regulatory guidelines, especially for pharmaceutical or food applications, prompt suppliers to follow global labeling norms like GHS pictograms or hazard statements. Concerns about workplace safety mean labels describe respiratory, skin, and environmental safety risks. Users demand tamper-proof seals and robust packaging to avoid cross-contamination and moisture ingress. Documentation includes not only safety data sheets but sometimes certificates for Good Manufacturing Practice (GMP) as extra assurance for critical-use customers.
Organic synthesis yields 3,4,5-trimethoxybenzoic acid from a few main starting points, such as methyl vanillate or gallic acid. Laboratories usually introduce methoxy groups through methylation, commonly by reacting gallic acid with dimethyl sulfate or methyl iodide in the presence of a base. Acidic or basic hydrolysis cleans up intermediate esters, freeing the carboxylic acid. Separation by crystallization and washing lets chemists zero in on high purity. Some industrial plants favor continuous flow reactors over batchwise glassware to drive scale and safety, substituting toxic reagents for greener ones where possible. Controls over reaction temperature, time, and solvent amount keep impurities down, helping both productivity and yield. Recovery and recycling of solvents matter too, as both cost and environmental compliance drive process improvements.
The carboxylic acid group on 3,4,5-trimethoxybenzoic acid drives many routes for further chemistry. Simple esterification with alcohols (using acid catalysts) produces esters that find use as intermediates in fragrance and pharmaceutical development. Amidation with amines yields amides for deeper medicinal chemistry campaigns. Reduction with strong hydrides like LiAlH4 brings out the alcohol (benzyl alcohol) form for downstream uses. The aromatic ring’s methoxy arms offer resistance to electrophilic attack, but careful demethylation can regenerate free phenol groups, offering even more ways forward for structural manipulation. Coupling reactions with activating agents like EDC or DCC enable peptide or nucleoside analogues, giving the acid added value for biological probe development. These transformations power both classic “library-building” and targeted synthesis, feeding into drug discovery, materials science, and biochemical exploration.
Looking for this compound by name brings up a variety of synonyms: Gallic acid trimethyl ether, Trimethyl gallic acid, and Benzoic acid, 3,4,5-trimethoxy-. Researchers sometimes refer to it by its registry number (CAS 86-81-7), avoiding mix-ups about positional isomers. In commercial catalogs, suppliers may brand the item or pack it under slightly altered trade names depending on the intended region or sector. Recognizing these synonyms helps buyers prevent costly order errors and ensures accurate regulatory reporting, especially in global operations. Regulatory filings and scientific texts benefit from standardized language, but a careful crosscheck of physical and chemical identifiers usually clears up any confusion.
Workplace exposure to 3,4,5-trimethoxybenzoic acid generally falls under the safety umbrella for aromatic acids. Laboratory and production staff should observe rules for handling chemicals with dust and fume hazards. Typical PPE includes gloves, goggles, and standard lab coats. Proper ventilation, splash-proof storage, and eye wash stations protect users from accidental exposure. Spills clean up best with inert absorbents, disposed of according to hazardous waste guidelines. Companies that care about regulatory compliance ensure that staff review both material safety data and emergency protocols as part of routine training. Long-term health studies on this compound remain limited, but analogous benzoic acid derivatives warrant respect due to their potential for mild skin or respiratory irritation. Environmental considerations have joined the regulatory checklist—dry, well-labeled containers, careful waste management, and avoidance of run-off into drains or soils all play crucial roles.
Workers in pharmaceutical R&D often reach for 3,4,5-trimethoxybenzoic acid because modifications at the methoxy positions can tune biological effects. Chemists designing anti-inflammatory drugs, antihistamines, or enzyme inhibitors sometimes use it as a lead structure. In agricultural chemical research, it serves as a tool for synthesizing analogues of naturally occurring plant defense molecules. Fragrance manufacturers explore its esters and derivatives for nonvolatile fixatives. More recent interest shows up in material science labs focused on constructing new aromatic polyesters and resins. The versatility of the acid allows for iterative design and optimization, essential traits for rapid prototyping and early-stage product discovery. Buyers paying attention to regulatory developments look for compounds with traceable provenance and robust safety profiles, and this acid answers that call for a growing swath of sectors.
Researchers exploring new therapies for diseases often examine chemical scaffolds based on 3,4,5-trimethoxybenzoic acid. The acid’s ease of functionalization lets medicinal chemists install side chains or link it to pharmacophores, chasing hits in high-throughput screening. Scientists also assess how its methoxy groups affect binding to biological receptors or change metabolic stability. Academic teams studying plant metabolism and secondary metabolites use the compound as a model molecule to tease apart biosynthetic pathways. Chemical biologists, focused on targeting specific enzymes with inhibitors or probes, appreciate the range of modifications 3,4,5-trimethoxybenzoic acid tolerates. Newer studies leverage computational chemistry to predict additional uses, saving time and resources before running expensive wet-lab experiments. Data sharing between groups accelerates the discovery cycle, fostering collaborative progress.
Toxicology studies of 3,4,5-trimethoxybenzoic acid draw comparisons to its less substituted cousin, benzoic acid, and its parent compound, gallic acid. Initial screenings in vitro suggest low acute toxicity, echoing results for related methoxybenzoic acids. Long-term studies are still sparse, so developers must rely on a blend of short-term animal exposures and predictive models. Preliminary evidence shows limited bioaccumulation, but researchers remain watchful for effects on aquatic species—a key factor for regulatory acceptance. Standard tests indicate the compound does not rapidly break down in soil or water, highlighting the need for careful disposal after use. Regulatory filings lean conservative until more definitive chronic exposure data comes in. Safety reviews in the literature urge laboratories to monitor for both allergic reactions and subtle respiratory effects, especially during larger preparations and repeated handling.
The outlook for 3,4,5-trimethoxybenzoic acid reflects the constant push for new molecules that connect academic insights to industrial application. Its functional versatility suggests future value in emerging fields such as targeted drug delivery, nanotechnology, and advanced polymer science. As green chemistry principles reshape how companies operate, demand rises for synthesis routes that reduce waste, lower toxicity, and use renewable inputs. Expect collaborations among academic, pharmaceutical, and chemical engineering teams to deliver fresh derivatives and improve existing process efficiency. Intellectual property filings already mention new uses, from specialty coatings to pro-drug candidates. Interest from regulatory agencies will only climb as more commercial applications come to market, so expect sharper focus on full lifecycle assessments—from cradle to grave—driven by both environmental policy and consumer demand for transparency.
Few chemicals manage to cover as much ground as 3,4,5-Trimethoxy Benzoic Acid in practical use. You find it on ingredient lists and research papers alike, mostly in pharmaceutical development. The reason often comes down to its molecular structure—a trio of methoxy groups attached to a benzoic acid backbone opens plenty of doors in synthesizing active substances. Over the years in the pharma industry, this compound kept showing up as a useful building block for antihypertensives and anti-inflammatories. Some folks even call it a core intermediate for synthesizing certain anticancer agents. In the lab, people value the predictable reactivity and versatility, seeing as it makes the path to new medicines a little less bumpy.
I’ve seen researchers lean on this compound for modifying existing drugs, looking for better patient outcomes. For example, 3,4,5-Trimethoxy Benzoic Acid forms the base for trimethoprim analogues and a range of histamine blockers. Research published in the Journal of Medicinal Chemistry points to its utility in fine-tuning how some molecules interact inside the body. While it doesn’t cure diseases by itself, it helps chemists get much closer to what patients need.
Work in the lab often calls for reliable intermediates, and this one earns trust as a go-to choice. It helps make esters and amides, which eventually become dyes, fragrances, and certain types of plastics. I met a chemist who swore by its use in making specialty flavors for processed foods—though the work happens mostly at the industrial scale, away from the consumer’s eye. Each time, chemists chase after predictable reactivity and manageable byproducts. This cuts down waste and boosts batch consistency, which matters if you’re making large quantities.
Some people might not recognize 3,4,5-Trimethoxy Benzoic Acid outside of scientific circles, but it supports the supply chain in understated ways. You find echoes of its influence from colors in synthetic textiles to stabilizers in everyday household products. Such applications show how chemistry quietly shapes modern life.
As with a lot of specialty chemicals, scaling up production while sticking to clean, green processes raises challenges. The traditional routes involve petrochemical sources, which stress the environment more than anyone likes to admit. Where I’ve seen promise: newer catalytic processes that cut down on harsh reagents. Teams working on bio-based synthesis have shown early success, though the costs still sit higher than tried-and-true petrochemical routes.
Worker safety also comes up, since continuous exposure in the plant poses health risks. I recall discussions around engineering controls and real-time air monitoring systems—simple changes that make a big difference. Attention to detail keeps hazardous exposure in check, while ongoing research looks for safer substitutes without losing the molecule’s benefits.
3,4,5-Trimethoxy Benzoic Acid built a solid reputation by consistently bridging gaps between raw chemistry and products people rely on. Demand from pharmaceutical labs likely won’t slow down, and fresh approaches to greener production could shift the whole industry forward. Those following the science can see a clear path: safer workspaces, cleaner manufacturing, and steady growth as chemists keep pushing for better solutions.
Anyone who’s handled 3,4,5-Trimethoxy Benzoic Acid in the lab knows that the listed purity spec isn’t some dry number tucked in a certificate. Purity means trust—whether you trust a reaction to finish right, or a reference standard to hold up under scrutiny. Most times, you’ll see manufacturers supplying this compound at a minimum purity of 98%. Some firms get bolder and promise 99% or even higher, but the magic number you see at most suppliers—98%—means they’re willing to stand behind it for research, synthesis, or pharma work.
Running a reaction with 3,4,5-Trimethoxy Benzoic Acid that isn’t as pure as claimed drops real headaches on your bench. Impurities steal yield, give rise to strange byproducts, or make spectra a mess to interpret. From my own time wrangling unfamiliar acids in small-scale synthesis, nobody wants to clean up a chromatogram that suddenly spawns extra peaks. A 98% spec doesn’t just cut down on those headaches—it brings some confidence that what you weigh out is what your chemistry expects.
Vendors don’t just pluck this number out of the air. They rely mostly on established techniques—high-performance liquid chromatography (HPLC) for clarity, melting point for a quick purity check, sometimes even titration. For 3,4,5-Trimethoxy Benzoic Acid, a sharp melting point (around 163-166°C for a pure sample) is an encouraging sign. HPLC traces need to show that one main component is towering above minor ones, so users see minimal unknown signals. In modern supply, GC-MS or NMR can back up claims, especially when guaranteeing the molecule’s structure—no one loves getting a surprise contaminant after spending weeks on a project.
Let’s be blunt—going below 98% creates extra risks. In pharma work, even tiny leftover organics or trace metals wreck a batch or mess with bioassays. Researchers focused on plant biochemistry or building new drug candidates rely on quality data. Any unexpected contaminant skews results, leading to wasted funds and lost time. Skipping proper purity checks makes a bad cycle: failed projects, frantic troubleshooting, budget overruns.
Labs can’t just take a vendor’s word for it. Good practice means requesting both a recent Certificate of Analysis and method sheets spelling out test specifics. Spot checks in-house, like running a melting point or quick NMR, keep vendors honest. Some labs even set up QC routines for every fresh batch—no shame in caution, since damage from a poor sample can snowball.
Beyond the numbers, look for suppliers who keep communication open—are they willing to share full spectra or chromatograms on request? Do they flag any changed lot numbers? Being able to contact a technical rep for clarity can rescue you from a batch of ambiguous results. In a world where speed and reliability shoulder more projects than ever, it’s never wasted effort to insist on solid, proven purity before trusting your next run.
I've worked with organic acids in both research settings and simple home labs, which means I've seen what goes wrong when storage gets sloppy. When talking about something like 3,4,5-Trimethoxy Benzoic Acid, carelessness can cause problems for you, your experiments, and anyone else who steps into your workspace.
This organic compound usually appears as a white to off-white powder. It’s pretty stable compared to many other reagents, but even stable chemicals can act up if you treat them carelessly. Excess moisture, light, and temperature swings can degrade solids or even trigger reactions with other substances floating around in the air. Accidental inhalation or contact won’t feel pleasant, either. Because of this, storage habits shape everything that comes after—from safety to the quality of your results.
Dry, cool, and well-ventilated describes the best kind of spot. Cabinets meant for chemicals at room temperature work better than drawers or workbenches. I keep my own benzoic acid derivatives in amber screw-cap bottles, tucked away inside a lockable metal storage cabinet. Light protection matters more than most people realize; visible and UV light mess with the molecular structure over time. Even fluorescent lights cause slow chemical changes that sneak up after a few months.
Avoid keeping acids close to bases or strong oxidizers. Mix-ups during a busy day can lead to spills that turn minor hassles into major headaches. I’ve had to deep-clean one too many benches thanks to carelessness—label shelves and make sure everything lines up by type.
Organic acids may not fizz on contact with water, but they clump and degrade if containers aren’t sealed tight. Humidity sneaks into loose lids, so always use containers with proper gaskets or liners. Toss in a silica gel packet for good measure if you don’t go through your stock very fast. Even a single humid afternoon can ruin an open container.
Glass, high-density polyethylene (HDPE), or polypropylene containers have held up best in my experience. Avoid using reactive metals or thin plastics—they leach or break down with time. Every bottle on my shelf gets labeled with the full name, concentration, and the date I opened it. More than once, I’ve caught myself before using an expired or contaminated chemical, thanks to these notes.
Solid storage routines go hand-in-hand with up-to-date records. Material safety data sheets (SDS) aren’t just paperwork to collect dust—read them before ever opening a container. Notes on shelf life, special handling tips, and disposal are there for a reason. I keep hard copies in a binder near the storage area and digital versions on my phone, so information follows me everywhere.
Each step in my process, from the container to the shelf label to the extra silica gel, came from cleaning up after mistakes—either my own or someone else’s. Smart storage of 3,4,5-Trimethoxy Benzoic Acid and other fine chemicals doesn’t have to be complicated. Good habits keep both people and projects protected, letting the science shine through safe and reliable results.
Years at the bench have shown me not every chemical with a scientific-sounding label sets off alarm bells. 3,4,5-Trimethoxy benzoic acid falls into a group of organic compounds used everywhere from pharmaceutical research to pigment production. Labs favor it for synthesis work. People question if it brings real risk or calls for the kind of heavy-duty protocols that come up with more notorious reagents.
A chemical safety sheet says a lot about what you’re dealing with—sometimes buried behind technical jargon. This compound shows low acute toxicity for skin and inhalation. In fact, manufacturers classify it as an “irritant.” Splash it in your eyes or leave it sitting on your skin and you’re asking for trouble: redness, stinging, maybe itching. Inhaling large amounts might cause mild nose or throat irritation. Swallowing a mouthful won’t feel good, but doesn’t usually lead to life-threatening danger for a healthy adult.
Despite that, the chemical world teaches not to drop guard. Even mild irritant powders still need gloves, goggles, and a working fume hood to keep any dust out of your lungs. No one in their right mind pours this into their coffee, sure, but sometimes accidents happen with small jars or quick weigh-ins. Proper labeling means nobody mistakes one white powder for another.
Respecting 3,4,5-Trimethoxy benzoic acid isn’t about treating it like cyanide or hydrofluoric acid. Instead, it’s the standard safety culture that avoids slip-ups. From my years in a teaching lab, newcomers get told: just because something isn’t fatal doesn’t give you license to ignore the rules. People get burns and rashes from “mild” acids and bases from simple mistakes, not just from gross negligence.
The other side has to do with scale. Handling a gram in a research experiment means a glass rod and a spatula do the trick. On an industrial kilo scale, fine powders dodge into the air and coat surfaces, turning a mild irritant into a workplace mess, especially if someone has allergies or asthma. Ventilation, masks, and cleanliness step from “nice to have” into “absolutely needed”.
Some of my former labmates had a saying: if you think a substance is harmless, you haven’t worked with it enough. 3,4,5-Trimethoxy benzoic acid isn’t in the same league as strong oxidizers or carcinogens, but it sits in the wide area between “harmless” and “hazardous waste.” That space relies on habit and a clear head. The compound can stain, irritate or make eyes water if you treat it like flour.
The drive for safer labs isn’t just red tape; a good safety record lets research run without disruption, lawsuits, or medical scares. Training, clear labels, and reachable spill kits matter. I’ve seen the difference when staff have a sense of ownership and watch out for each other, compared to the rushed “that’ll do” attitude where small messes build up to real problems over time.
Don’t skip gloves or goggles, even if you know the MSDS by heart. Use a fume hood when weighing or transferring. Wash up after handling. Store the jar away from food prep, strong bases, or oxidizers so you don’t end up with an unwanted reaction. Dispose of waste following recommended routes—never down the drain or casually in office bins.
A little respect, the right gear, and clear habits keep 3,4,5-Trimethoxy benzoic acid in the “routine chemical” column, not a headline-maker.
Lab researchers and quality managers see this question pop up every week: “Can you provide the certificate of analysis for 3,4,5-Trimethoxy Benzoic Acid?” The answer shouldn’t just be a yes-or-no affair. Behind this request, there’s a lot more than protocol. That certificate speaks volumes about the way a supplier manages risk, quality, and even honesty.
In the pharmaceutical and research world, small mistakes can snowball. Years ago, while running a synthesis project, I learned the hard way how an unreliable certificate could send weeks of work into the trash. Even a trace level of an extra impurity, missed by unreliable suppliers, can turn an entire effort into scrap. Knowing who vouches for their powder isn’t an optional comfort; it keeps projects on schedule and protects both reputations and bottom lines.
A credible COA doesn’t hide behind jargon. It lists identification, purity, related substances, water content, and the analytical methods behind those numbers, usually HPLC or NMR. Every lot number links directly to these results, tying quality claims to a real sample someone actually tested. If you spot vague purity claims or generic tables without batch IDs, that’s a sign to ask harder questions. From my experience, labs that invest in full-spectrum analysis avoid the headaches created by unverified or incomplete results.
Regulation matters, but personal standards run even higher. The COA stands as an accountability tool. It’s not just about satisfying auditors from the FDA, EMA, or local agencies. It ensures proper science and, in the end, public safety. Remember melamine in milk or heparin scandals? Skimping on method verification put lives at risk. A good certificate won’t let something like this slip through the cracks. The stakes remain high for anyone beyond the academic lab: manufacturing, dietary supplement producers, clinical investigation teams—all depend on the numbers behind the label.
Demand more than the minimum. Remind suppliers that you expect transparency, not only for current good manufacturing practice (cGMP) compliance but as a basic condition for business. I’ve seen organizations quietly switch vendors just because one provided a chromatogram or mass spectrum with their COA and another simply uploaded a PDF with minimal numbers. Rewarding thorough documentation increases the odds that rigorous habits spread throughout the industry.
It pays to train every lab member to read certificates critically, not just file them. Basic training in GMP or laboratory documentation opens eyes. When suppliers know you check retention time, peak purity, and identity by comparison to known standards, corners stop getting cut. This is one way to put E-E-A-T principles—experience, expertise, authority, and trust—into daily practice, not just policy documents.
Certificates of analysis aren’t hurdles. They cost a few minutes upfront but save months of clean-up later. For anyone handling 3,4,5-Trimethoxy Benzoic Acid or any chemical where purity, identification, and trace properties shape the experiment, the COA isn’t just paperwork. It’s insurance, and it’s peace of mind backed by data, real work, and real-world consequences.
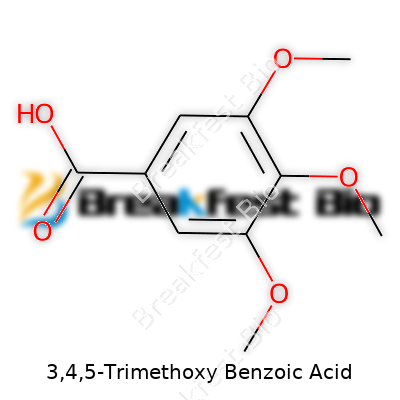
| Names | |
| Preferred IUPAC name | 3,4,5-Trimethoxybenzoic acid |
| Other names |
3,4,5-TMBA
Benzoic acid, 3,4,5-trimethoxy- Gallic acid trimethyl ether m-Trimethoxybenzoic acid Trimethyl gallic acid |
| Pronunciation | /ˌtraɪˌmɛθˈɒksi bɛnˈzoʊɪk ˈæsɪd/ |
| Identifiers | |
| CAS Number | 86-81-7 |
| Beilstein Reference | 105889 |
| ChEBI | CHEBI:9048 |
| ChEMBL | CHEMBL15972 |
| ChemSpider | 2354 |
| DrugBank | DB04250 |
| ECHA InfoCard | 03fa1e20-bc5b-4051-8a5f-b7baa999cbe2 |
| EC Number | 2076-56-0 |
| Gmelin Reference | 8428 |
| KEGG | C06723 |
| MeSH | D013678 |
| PubChem CID | 6919 |
| RTECS number | VO2450000 |
| UNII | G96K7WI48A |
| UN number | UN3077 |
| CompTox Dashboard (EPA) | DTXSID6020281 |
| Properties | |
| Chemical formula | C10H10O5 |
| Molar mass | 212.19 g/mol |
| Appearance | White to Off-white solid |
| Odor | Odorless |
| Density | 1.347 g/cm³ |
| Solubility in water | slightly soluble |
| log P | 1.69 |
| Acidity (pKa) | pKa = 4.19 |
| Basicity (pKb) | 11.85 |
| Magnetic susceptibility (χ) | -62.5 × 10⁻⁶ cm³/mol |
| Refractive index (nD) | 1.568 |
| Viscosity | Viscous liquid |
| Dipole moment | 2.42 D |
| Thermochemistry | |
| Std molar entropy (S⦵298) | 224.2 J·mol⁻¹·K⁻¹ |
| Std enthalpy of formation (ΔfH⦵298) | -631.6 kJ/mol |
| Std enthalpy of combustion (ΔcH⦵298) | -1709.8 kJ/mol |
| Pharmacology | |
| ATC code | A01AA06 |
| Hazards | |
| Main hazards | Harmful if swallowed; causes skin and eye irritation. |
| GHS labelling | GHS07, GHS05 |
| Pictograms | GHS07 |
| Signal word | Warning |
| Hazard statements | H315, H319 |
| Precautionary statements | P261, P264, P271, P272, P273, P280, P301+P312, P330, P501 |
| NFPA 704 (fire diamond) | 1-1-0-0 |
| Flash point | Flash point: >110°C |
| Autoignition temperature | Autoignition temperature: 450°C |
| Lethal dose or concentration | LD50 oral rat 3900 mg/kg |
| LD50 (median dose) | LD50 (median dose): Oral, rat = 5,000 mg/kg |
| NIOSH | DH6650000 |
| PEL (Permissible) | 10 mg/m3 |
| REL (Recommended) | 10 mg |
| IDLH (Immediate danger) | NIOSH has not established an IDLH value for 3,4,5-Trimethoxy Benzoic Acid. |
| Related compounds | |
| Related compounds |
Gallic acid
Vanillic acid Protocatechuic acid Syringic acid 3,4,5-Trimethoxybenzaldehyde 3,4,5-Trimethoxybenzyl alcohol Methyl 3,4,5-trimethoxybenzoate 3,4,5-Trimethoxytoluene |